Soil is any unwanted matter on the surface of an object that one desires to be clean. Cleanliness is an unnatural condition, because all surfaces are constantly being soiled. In order to clean a surface (substrate), it is, therefore, necessary to work against nature, and special care must be taken to ensure that all soil is removed and that it is not redeposited on the substrate. This section deals with classes of soils and the identification of them.
Soil may be classified as visible and invisible, the latter category being primarily micro-organisms, such as bacteria, yeasts and moulds. Soil is best identified by characteristics that give information on how it may be solubilised, because the object of cleaning is to dissolve or to suspend soil and then to wash it away.
Visible soil is classified according to its solubility characteristics — whether it is:
- Soluble in water
- Soluble in alkali
- Soluble in acid
- Soluble in surfactant solution
- Insoluble in any of the above
Soils, such as sugar, and some inorganic compounds like ordinary salt can be dissolved and washed away by ordinary water. In addition, the greater part of food soil can either by suspended in water or can simply be removed from a surface by the force of a water spray. Any soils not directly soluble in water will be left behind as a thin film or as a deposit.
Some of these films and deposits are either solubilised or emulsified by alkaline detergent solutions. Natural fats and oils from plants and animals contain small amounts of free fatty acids. These acids are neutralised by alkaline solutions forming small amounts of soluble soap. This soap can then aid in the emulsification of the fats and oils that are present in many soils or films. Alkalis also react directly with plant and animal fats and oils to form soap, glycerine, and water by a process called saponification. Neutralisation is a much more important reaction than saponification in the removal of greasy films, however, because it is a fast reaction as compared to saponification.
ContentsProtein soils from milk, eggs, meat etc., can also be solubilised by alkaline solutions. Proteins will hydrate and swell when they come into contact with water which helps alkalis to react with them, forming soluble salts. To a small degree the protein soils may also be broken down into smaller, more soluble molecules by a reaction with alkaline materials which is called peptization. This reaction is slow, however, and therefore, relatively unimportant.
ContentsMilkstone, mineral deposits and other deposits formed by the reaction of minerals with organic substances often are deposited on surfaces where they are not wanted. This type of soil is sometimes very complex, but at least part of it will be solubilised by a reaction with acid. Even if only a part of the soil is removed in this manner, the remainder is often loosened during the reaction so that it is washed away. In those cases where part of the deposit is composed of fat, the acid can be followed by an alkaline wash.
ContentsLubricating greases and oils are not solubilised by either acids or alkalis. They can sometimes be melted by hot water or steam but even then, a residue is often left behind. Surfactants help emulsify this material by breaking it up into small globules which are soluble in water or which can be suspended in water. Most dust and dirt contains some oily material which makes removal by this method possible.
ContentsThose soils which are insoluble in water, in alkaline solutions, or in acid solutions usually do not cling to most surfaces anyway. Surfactants often allow the detergent solution to wet these soils so that they can be suspended in water or so that they can be flushed from the surface to be cleaned. Examples of such soils are sand, clay and fine metal particles. Charred or carbonised soil is more difficult to remove.
During the processes in which water is removed from food and in pasteurisation and sterilisation heat is often applied in a manner that chars or carbonises some of the food. The chemistry is often complex, but in ordinary physical terms this is simply burned-on food. A 1% caustic solution applied at 180°F (82.2°C) with brushing will usually solubilise or suspend this type of soil.
ContentsChemicals and basic directions for the identification of most soils....
It is important (especially when one is not yet completely familiar with soil testing) to perform the tests in the order given here. This will eliminate unnecessary testing and also avoid incorrect soil identification.
ContentsPlace a drop or two of phenolphthalein on the soil. If a pink colour appears, the soil is alkaline; if there is no colour change, the soil is neutral or acid. Most alkaline residues are caused by poor rinsing after washing with an alkaline detergent, but sometimes they are caused by alkaline water supplies. The latter case can easily be verified by checking the pH of the raw water. If it is over 9, the cause may very well be the water. The majority of food soils should be neutral or acid in nature.
ContentsThe preceding test may have indicated alkalinity by turning phenolphthalein pink. This test will tell whether an alkaline soil can be removed completely by acid. Add a few drops of acid as indicated in the directions, allow to stand for at least two minutes, and then rinse with water. If the soil is completely removed from the spot, an acid product will remove the soil. If only part of the soil is removed, other treatment is required.
ContentsAdd a few drops of caustic to the soil, allow to stand for at least two minutes, and rinse with water. If the soil has been removed, an alkaline product can be recommended. If only a part of the soil was removed, further testing and treatment is necessary.
ContentsAdd a few drops of caustic and drop of two of Hi Tech Detergents XY-13 (Sodium Hypochlorite) to the soil. Allow to stand for at least two minutes and rinse with water. If this test removes soil that was only partially removed in the last test, a chlorinated alkaline cleaner is required.
ContentsIf the tests for alkaline, acid and protein soils do not remove the film or soil, then and only then should the test for silica film be run. Place a few drops of silica reagent on the soil and allow it to stand for at least two minutes before rinsing it off. If soil is removed that could not be removed by any other test, there is a possibility that there is a silica film. A special acid treatment is needed to remove it, but this treatment is dangerous and should never be used without the authorisation of your supervisor.
The foregoing tests with their recommendations will help in solving many cleaning problems even if the approach to testing has been over simplified. The examples given here illustrate the manner in which these tests can be combined for the solution of some complex as well as some simple soil problems.
ContentsA series of tests have been run on a soil with the following results....
| Phenolphthalein | Colourless |
| Acid | No reaction |
| Caustic | Some reaction after two minutes |
This indicates a neutral or acid type soil, such as fat, protein, or both. A longer test with caustic should be run to see if the soil can be completely removed or if further testing is required. Product selection should be made after that.
In another example, the test results were as follows:
| Phenolphthalein | Colourless |
| Acid | Slight reaction |
| Caustic | Removal of part soil. When tried on the same spot as the acid test, complete removal of soil. |
This indicates that there is fat, protein, or both in the soil as well as a slight mineral deposit. The way the tests were run indicates that an acid wash followed by an alkaline product would be effective in removing the soil. It may be possible to reverse the order for the same result.
Quite often, a soil that is slowly, but completely removed by caustic alone will be removed much faster by caustic and chlorine. The technician must take time difference into account when choosing between an alkaline and a chlorinated alkaline product.
A special note on the test for silica film is necessary here to avoid the unnecessary use of this special reagent.
- silica film is quite rare.
- this reagent will remove almost any soil.
- this reagent is dangerous to handle — extremely so.
Only if one is absolutely positive that no other means are available for the removal of a soil, should one request this chemical.
ContentsThe soil chart which follows lists some common films and deposits, their causes, their removal and their prevention.
A well designed cleaning system should have trouble-free operation as one of its goals, and if it is maintained properly, it should function for long periods of time without problems. However, any given system may have a flaw in it; part of the cleaning procedure may be omitted at times; the equipment may break down; the water supply may change; etc. Any of these "errors" or changes may lead to the formation of a film or a deposit. The table lists and describes some films and deposits, their probable causes, and some methods for their removal. The prevention of further problems will be obtained by adhering to proper cleaning and maintenance procedures.
| Film or Deposit Identification | Description | Probable Causes | Procedure for Removal |
| Protein Film | A blue or rainbow coloured film having a varnish-like appearance similar to dried apple sauce. | Use of a non-chlorinated cleaner. Inadequate pre-rinse. Periodic instead of regular cleaning. | Make a paste with equal parts of chlorinated cleaner, alkaline cleaner and water, and apply this to the soil. Allow to soak and then wash with water. |
| Fat, Grease, or Oil Film | A greasy, oily, sometimes white film on which water forms into beads. | Use of an acid product for washing. Low wash temperature. Oil from equipment. | Wash with hot, alkaline surfactant solution. |
| Factory Soil | A black and/or greasy film. | Oil and dirt from the manufacturing process. Grease or oil coating for protection during storage. | Wash with hot, foamy, alkaline detergent solution. If rusty, wash with and acid product. |
| Surfactant Film | A blue film. | Poor rinsing. | Wash with a hot detergent solution. Brushing may be necessary. |
| Food Stabiliser Film | A white, sandy deposit. | Adherence of food stabilisers from foods such as cheese, ice-cream, convenience foods, etc., when only alkaline cleaners are used. | Wash with an acid solution. |
| Rubber Film | Black streaks or a film which may be sticky. | Reaction of rubber with a chlorinated product or ageing of rubber. | Wash with an acid solution and replace rubber parts that are sticky or that still have black streaks. |
| Silica Film(very rare) | A white or grey glaze. | Silica from a water supply when there is poor rinsing or when mechanical cleaning is used where manual cleaning is specified. | Clean with a special acid wash (This acid is very dangerous and cannot be used without the proper approval). |
| Mineral Deposit | A white, grey or yellow deposit such as milkstone, beerstone, waterstone etc. | Minerals in water settling out or reacting with substances in milk, beer, meat, fruit and then settling out. | Wash with an acid product. In breweries a solution of EDTA in water is often used to remove beerstone. |
| Iron Deposit | A red, brown or black deposit. | High iron content in the water supply or iron from system components and a lack of iron removal equipment. | Wash with an acid product or with a 5% citric acid solution. |
| Corrosion | A rusty or pitted surface. | Migrating metal particles or excessive contact time with a sanitising rinse. | Wash with an acid product and brushing to remove rust. Repolish and passivate pitted surface. |
| Corrosion | A black residue or deposit. | Contact of two different metals such as two types of stainless steel. Chemical action of alkaline cleaner or aluminium. | Wash with an acid product. |
| Corrosion | A blue to black film on stainless steel in high temperature equipment. | Oxidation through foaming or aeration under conditions of high alkalinity and high temperature. | Treat with potassium permanganate and phosphoric acid |
| Etching | Pitting, usually with a white deposit on the pits. | Use of improper chemicals or failure to use chemicals correctly. | Repolish and passivate pitted surface. |
| The conditions listed below pertain to plastic materials, usually tubing, that are ordinarily clear and colourless. All of them can be prevented or postponed by careful adherence to good cleaning procedures. However, all plastic must be replaced eventually. |
| Opaque condition | Plastic is no longer clear and may appear white. | Absorption of moisture due to poor drainage or a lack of drying. | Expose to heat and light (sunlight). Forced air drying may be necessary. |
| Yellowing | Gradual formation of yellow discoloration. | Ageing of plastic or improper use of an iodophor. | Cannot be removed. Replace the plastic. |
| Brown or Black Film | Brown or black deposit may appear as specs, steaks or film may appear suddenly or gradually. | Migration of rubber particles or carbon particles from motors. | Wash with acid solution. Replace the plastic if washing does not remove the film. |
| Red Stain | Bacterial pigment. | Pigment from the organism Serratia marcescens. | No procedure is known for the removal of this stain. |
| Pink or Purple Stain | Bacterial pigment. | Pigment from the organism, Streptococcus rubrireticuli. | Wash with a highly alkaline solution.
|



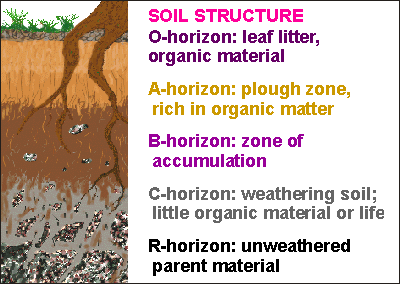 Whereas soil is formed from the rock below, it is eroded away from the top. A cover of plant life slows down erosion, allowing the soil layer to build up, but there is more going on.
Whereas soil is formed from the rock below, it is eroded away from the top. A cover of plant life slows down erosion, allowing the soil layer to build up, but there is more going on.

 polyethylene glycol chain (Figure Three). In each case, the hydrophilic end of the surfactant is strongly attracted to the water molecules and the force of attraction between the hydrophobe and water is only slight. As a result, the surfactant molecules align themselves at the surface and
polyethylene glycol chain (Figure Three). In each case, the hydrophilic end of the surfactant is strongly attracted to the water molecules and the force of attraction between the hydrophobe and water is only slight. As a result, the surfactant molecules align themselves at the surface and  internally so that the hydrophile end is toward the water and the hydrophobe is squeezed away from the water (Figure Four).
internally so that the hydrophile end is toward the water and the hydrophobe is squeezed away from the water (Figure Four). The surface to be cleaned and the soil to be removed must initially be wet and the soils suspended, solubilised, dissolved or separated in some way so that the soil will not just re-deposit on the surface in question (Figure Five).
The surface to be cleaned and the soil to be removed must initially be wet and the soils suspended, solubilised, dissolved or separated in some way so that the soil will not just re-deposit on the surface in question (Figure Five).