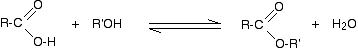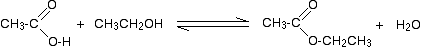Halaman ini menjelaskan tentang reaksi aldehid dan keton dengan 2,4-dinitrofenilhidrazin (pereaksi Brady) sebagai sebuah reaksi uji untuk ikatan rangkap C=O. Disini kita juga membahas sekilas tentang beberapa reaksi mirip lainnya yang dikenal sebagai reaksi adisi-eliminasi (atau kondensasi).
Reaksi dengan 2,4-dinitrofenilhidrazin
2,4-dinitrofenilhidrazin sering disingkat menjadi 2,4-DNP atau 2,4-DNPH. Larutan 2,4-dinitrofenilhidrazin dalam sebuah campuran metanol dan asam sulfat dikenal sebagai pereaksi Brady.
Pengertian 2,4-dinitrofenilhidrazin
Walaupun namanya kedengaran rumit, dan strukturnya terlihat agak kompleks, namun sebenarnya sangat mudah untuk dibuat.
Pertama-tama gambarkan rumus molekul dari hidrazin, yaitu sebagai berikut:
 Pada fenilhidrazin, salah satu atom hidrogen dalam hidrazin digantikan oleh sebuah gugus fenil, C6H5. Ini didasarkan pada sebuah cincin benzen.
Pada fenilhidrazin, salah satu atom hidrogen dalam hidrazin digantikan oleh sebuah gugus fenil, C6H5. Ini didasarkan pada sebuah cincin benzen.
 Pada 2,4-dinitrofenilhidrazin, ada dua gugus nitro, NO2, yang terikat pada gugus fenil di posisi karbon 2 dan 4. Sudut yang padanya terikat nitrogen dianggap sebagai atom karbon nomor 1, dan perhitungan dilakukan searah arah jarum jam.
Pada 2,4-dinitrofenilhidrazin, ada dua gugus nitro, NO2, yang terikat pada gugus fenil di posisi karbon 2 dan 4. Sudut yang padanya terikat nitrogen dianggap sebagai atom karbon nomor 1, dan perhitungan dilakukan searah arah jarum jam.

Reaksi dengan 2,4-dinitrofenilhidrazin
2,4-dinitrofenilhidrazin sering disingkat menjadi 2,4-DNP atau 2,4-DNPH. Larutan 2,4-dinitrofenilhidrazin dalam sebuah campuran metanol dan asam sulfat dikenal sebagai pereaksi Brady.
Pengertian 2,4-dinitrofenilhidrazin
Walaupun namanya kedengaran rumit, dan strukturnya terlihat agak kompleks, namun sebenarnya sangat mudah untuk dibuat.
Pertama-tama gambarkan rumus molekul dari hidrazin, yaitu sebagai berikut: